
Aptio Group specializes in regulated industries, offering consulting solutions across pharmaceuticals, medical technology, biotechnology, cosmetics, food technology, automotive, and recruitment services.
At Aptio Group, we strive to be trusted advisors, rather than just a supplier. Our tailored, cost-efficient solutions meet today’s regulatory demands, and our comprehensive support spans the entire value chain, guiding projects from inception to completion. Our focus is delivering excellence, providing our customers with solutions and services viewed as investments rather than costs and being the trusted advisor to support your success.
Our consultants have a mix of seniority and specialized expertise, ensuring we provide the right talent and strategic guidance to support your business growth. Driven by continuous learning and problem-solving, we foster a culture of ownership and responsibility, leveraging our employees’ knowledge, ideas and creativity to shape a sustainable world.
We offer flexible and reliable solutions, from short-term engagements to full-time contracts, ensuring the best fit for our customers.
Together, we’ll ensure compliance today while empowering innovation for tomorrow.

We offer specialist expertise to the pharmaceutical and biotech industries, covering the entire drug development chain—from R&D to clinical studies, manufacturing, and commercialization. We collaborate with companies of all sizes, providing the expertise needed for success. Through consulting, recruitment, and management services, we deliver tailored solutions to meet the unique challenges of regulated industries, ensuring compliance and operational excellence. This may, for example, but not exclusively consist of support within;
· Leadership
· Scientific Roles
· Manufacturing & Production
· Product & Marketing
· Clinical & Medical Affairs
· Quality, Regulatory, and Compliance
At Aptio Group, we focus on the medical device sector, delivering specialized expertise from research and development all the way to market launch. Our customized solutions and professional support simplify certification and help meet all regulatory demands. Through consulting, recruitment, and management services, we deliver tailored solutions to meet the unique challenges of regulated industries, ensuring compliance and operational excellence. This may, for example, but not exclusively consist of support within;

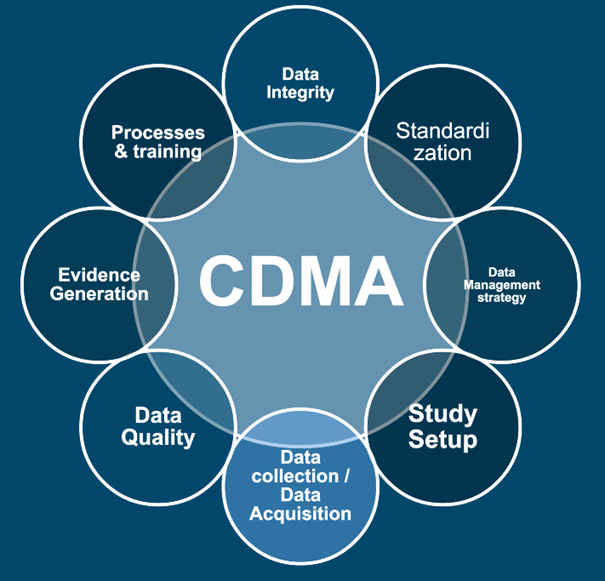
In a fast-evolving landscape, robust data strategies are essential for staying ahead. At Aptio Group, our senior experts support you throughout the entire process—from study setup to delivering analytics-driven insights.
Clinical data is a goldmine, yet unlocking its full potential remains a challenge for many companies. At Aptio Group, we offer tailored solutions to help you set up studies, collect data the right way, and generate evidence to support informed decisions. By integrating insights from diverse sources, we ensure your data works for you—enabling stronger claims and superior outcome.

At Aptio Group, we specialize in the food and cosmetics industries, leveraging deep expertise to drive sustainable business growth. Through consulting and strategic recruitment, we support research, innovation, production, and business development. We collaborate with businesses of all sizes, ensuring they have the resources and expertise to thrive.
This may, but not exclusively, consist of support within:
· Research & Scientific Roles
· Quality, Regulatory and Compliance
Aptio Goup offers specialized expertise within the automotive industry, combining industry-specific knowledge with a client-centered approach.
At Aptio Group, we possess the skills and capabilities to provide comprehensive services across all areas of the automotive sector.


We provide training in several areas that concern how to comply with and relate to current regulations and practices, primarily in the pharmaceutical, medical device, automotive and food industries.
We can also customize training and seminars to our customers’ purposes and needs. We offer to customize training according to customers’ needs in a wide perspective.
This may, for example, but not exclusively consist of:







Aptio Group is the leading company that delivers highly qualified services to customers with regulatory…